
The new veterinary medical products law
This article refers to a publication by the “Deutsche Tierärztliche Wochenschrift”, which is a journal of the German Veterinary Medical Association. The publication provides an overview of the new veterinary medical products regulation that came into force in January 2022 and its implementation in Germany. The new veterinary medical products regulation is of relevance as it inter alia regulates the marketing authorisation of products containing biomaterials such as novel therapy veterinary medicinal products.
The previously relevant regulations on medical products were replaced by the new European veterinary medical products legislation on 28.01.2022 [1]. The new legislation consists of the following regulations:
- Regulation (EU) 2019/6 on veterinary medical products
- Regulation (EU) 2019/4 on the manufacturing, placing on the market and use of medicated feed, and
- Regulation (EU) 2019/5 laying down Community procedures for the authorization and supervision of medical products for human and veterinary use.
In this context, Regulation (EU) 2019/6 on veterinary medical products replaced Directive 2001/82/EC on the Community code relating to veterinary medical products. This change of legal act – from directive to regulation – results in a new legal system, since a directive defines a goal to be achieved by all EU countries, for the realization of which the individual EU member states enact their own legal provisions. In Germany, this took place primarily in the German Medicines Act (AMG) and its subordinate legislation, such as the Ordinance on Veterinary Home Pharmacies (TÄHAV). A regulation is a binding legal act that is directly applicable in all member states. This means that national implementation is not required for the final provisions regulated in the regulation. However, Regulation (EU) 2019/6 does not regulate all aspects of veterinary medical product law, but leaves the member states the option of regulating certain matters at national level. This is the case, for example, with the retail sale of veterinary medical products, so that the veterinary dispensing right could be retained in Germany, even if it does not exist in all member states. However, this national regulatory leeway also means that German legislation on veterinary medical product law will continue to exist alongside Regulation (EU) 2019/6 (Fig. 1). Since the German legislator was of the opinion that, due to the future differences between the provisions for veterinary and human medical products, it is not feasible to continue to regulate the legal areas together in the AMG, a new Veterinary Medicinal Products Act (TAMG) was introduced [1].
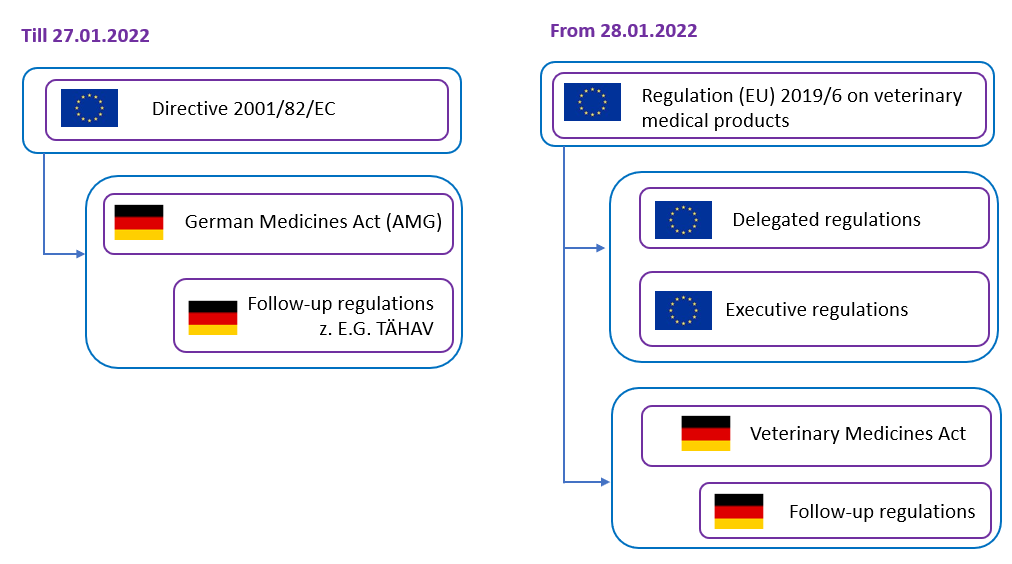
The EU Regulation (EU) 2019/6 [2] on veterinary medicinal products explains the definitions and classifications of veterinary medical products, including those that contain biomaterials, such as immunological products, or novel therapy veterinary medicinal products, which also encompass products that consist of engineered tissues or cells. It also discusses the requirements and procedures for the authorization, distribution, and pharmacovigilance of these products, as well as the responsibilities and obligations of the veterinarians, manufacturers, and distributors.
References
[1] Emmerich I, Sommerhäuser J (2022) , Das neue Tierarzneimittelrecht, Bundestierärztekammer, https://bundestieraerztekammer.de/btk/dtbl/archiv/2022/artikel/DTBl_01_2022_Tierarzneimittelrecht.pdf, Accessed on 02.10.2023
[2] The European Parliament and the Council of the European Union, Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/E. 2022. [Online]. Available: https://eur-lex.europa.eu/eli/reg/2019/6/oj
Keywords
veterinary medical products, law, use of biomaterials, requirements, procedures