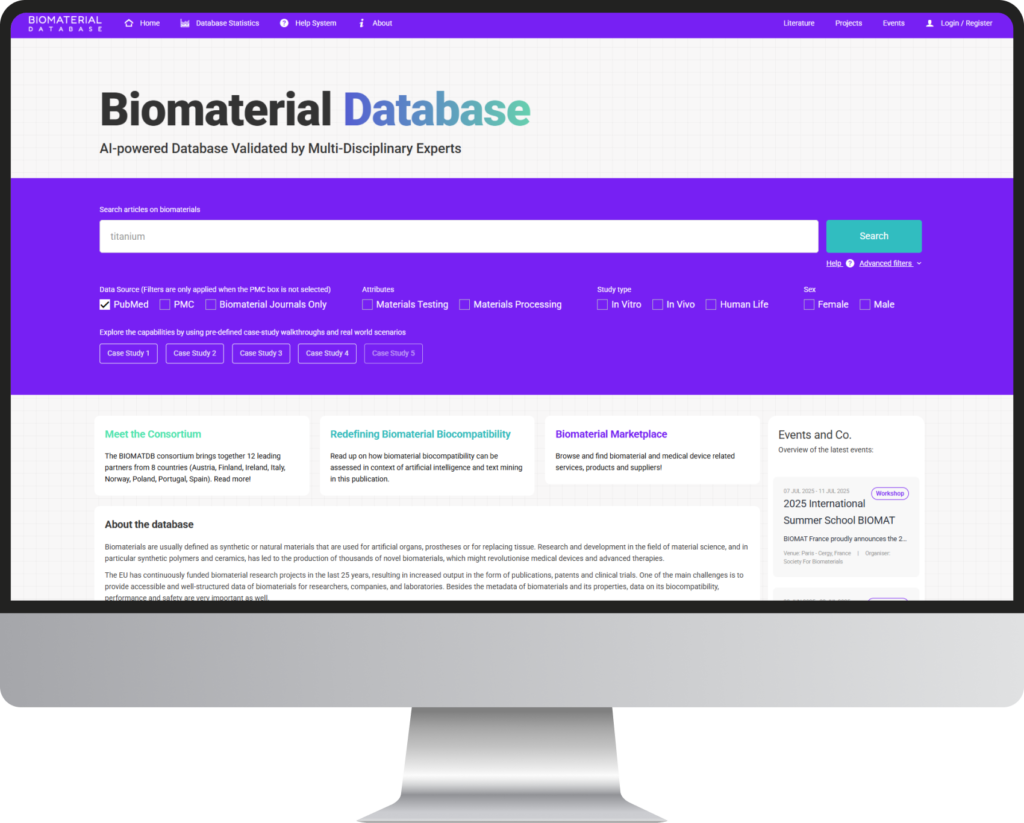
Biomaterials are usually defined as synthetic or natural materials that are used for artificial organs, prostheses or for replacing tissue. Research and development in the field of material science, and in particular synthetic polymers and ceramics, has led to the production of thousands of novel biomaterials, which might revolutionise medical devices and advanced therapies.
The EU has continuously funded biomaterial research projects in the last 25 years, resulting in increased output in the form of publications, patents and clinical trials. One of the main challenges is to provide accessible and well-structured data of biomaterials for researchers, companies, and laboratories. Besides the metadata of biomaterials and its properties, data on its biocompatibility, performance and safety are very important as well.
ASSESS existing knowledge on biomaterials to identify best practices for material databases as well as survey relevant stakeholders to gather requirements
DEVELOP an advanced database of biomaterials providing detailed information of all relevant properties and intuitive data analysis and visualisation for a variety of end users
LAUNCH of an information marketplace for suppliers of biomaterials and implementation of digital advisors to support demanders with a guided step-by-step product search
CREATE a label of biocompatibility to define the suitability of biomaterials for use in medical devices or advanced therapies to assist companies and SMEs to access markets
DEMONSTRATE the database and marketplace following structured test and validation cycles in combination with training activities and derive a feasible business model
INCREASE awareness and impact through effective and measurable communication and dissemination activities on the purpose of the project and the developed solutions
Duration
06/2022 to 11/2024
Programme
Horizon Europe
CL4-2021-RESILIENCE-01-25
Coordination & Support Action
Reference
101058779
Project Methodology

ADVANCED DATABASE & DATA ANALYSIS AND VISUALISATION TOOLS
BIOMATDB focuses on the creation of an advanced Biomaterial Database, providing detailed information on the chemical-physical, biological and toxicological properties accessible to a wide variety of end-users such as researchers, companies, clinicians, etc. The database accessible via www.biomaterialdatabase.com will be built with a modern online user frontend (web platform) and will also include several data analysis and visualisation tools making use of enhanced data processing technologies.

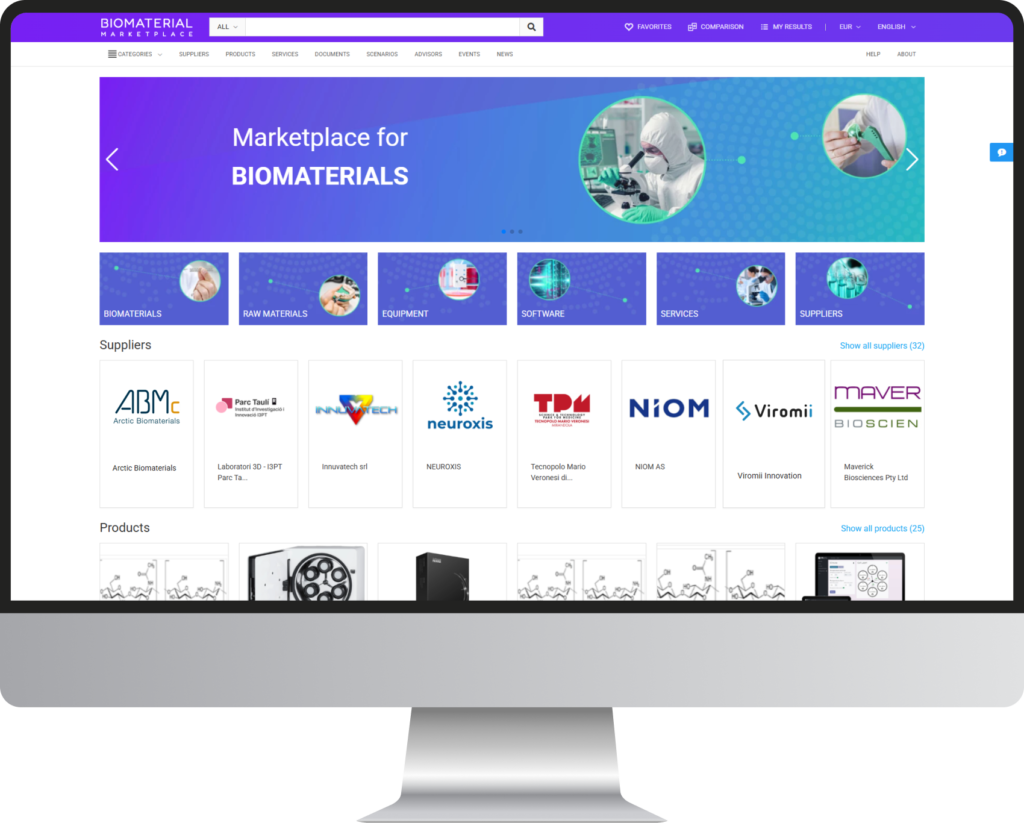
INFORMATION MARKETPLACE & DIGITAL ADVISORS
BIOMATDB additionally assists companies, especially SMEs, in facilitating market access for their products. Thus, the Biomaterial Database will be extended by an information-oriented Biomaterial Marketplace accessible via www.biomaterialmarketplace.com. The marketplace connects the suppliers and demanders on a user-friendly and informative online web application instance. Integrated Digital Advisors support a step-by-step guided search.

LABEL OF BIOCOMPATABILITY FOR BIOMATERIALS
BIOMATDB focuses on the creation of a label of biocompatibility to define the suitability of a biomaterial for use in a medical device or advanced therapy and to assist companies, especially SMEs, in choosing and facilitating market access for their products. The label of biocompatibility will be based on the main steps foreseen in the ISO 10993, which provides a framework for evaluation the biological safety of medical devices based on pre-clinical studies, and on clinical data.
Project Impacts

Improved access to valuable data and tools by linking to external resources for cross disciplinary data exploitation and integration

Domain specific text mining, data analysis, visualisation tools and decision support mechanisms in context of biocompatibility

Improved biomaterials research, development and exploitation cost-effectiveness for applications through shared biomaterials knowledge bases
Economic impact based on an extensive data pool with deep analysis capabilities and advanced data visualisation options

Raising awareness on sharing and big data awareness and interest among the biomaterial research community and industry

Support for the suppliers of biomaterials (companies, especially SMEs) in choosing and facilitating market access

Increase the public acceptance of Artificial Intelligence and related modern technologies and its benefits
Impact on the competitiveness of the European Union in the field of biomaterials, medical devices and biomedical engineering
Improved access to valuable data and tools to versatile end users and by linking to external resources for cross disciplinary data exploitation and integration
The BIOMATDB platform is expected to have a clear scientific impact advancing the field of biomaterials by providing a new open-access information resource to organize knowledge, facilitate systematic information search and enable evidence-based selection of materials for medical applications and medical device design. As an open access tool, the database will be accessible stakeholders in less privileged settings.


Domain specific text mining, data analysis and visualisation tools and decision support mechanisms in context of biocompatibility
The various tools generated in the duration of the project (ex: ontologies, annotation configurations) with specific utility in the biomaterials and medical device-related literature will benchmark and enable easier information extraction by anyone interested, and are likely to rapidly advance this effort. Future approaches will benefit from easily available, open-source pipelines and tools as well as enable better decision support for doctors and even patients, when choosing a medical implant.
Improved biomaterials research, development and exploitation cost-effectiveness for applications through shared biomaterials knowledge bases.
BIOMATDB will unlock currently inaccessible biomaterials data in unstructured data and allow marketplace data use. Effective use of this broad and deep information asset will improve existing and derive new AI biomaterials data reuse. Deployment of these solutions improve the knowledge about existing biomaterials properties used in clinical practice as well as novel biomaterials in research and development community. This could impact the economic development of companies, especially SMEs, of the member states, by providing better insights to promote the development and characterization in the clinical settings of biomaterials as well as more comprehensive view of their properties.
Economic impact based on an extensive data pool with deep analysis capabilities and advanced data visualisation options
From a commercial point of view, an extensive dataset will allow a deep analysis of the outcomes of translation in the field of biomaterials from early research phases through to patenting and clinical trials, and could help to identify failures along the translation pipeline, recognize areas of strength and weakness, and provide insights for scientists, organisations and policy makers. Moreover, patenting data is expected to have high value for industry, and facilitate attracting interest and further funding.
Improved biomaterials research, development and exploitation cost-effectiveness for applications through shared biomaterials knowledge bases.
BIOMATDB will unlock currently inaccessible biomaterials data in unstructured data and allow marketplace data use. Effective use of this broad and deep information asset will improve existing and derive new AI biomaterials data reuse. Deployment of these solutions improve the knowledge about existing biomaterials properties used in clinical practice as well as novel biomaterials in research and development community. This could impact the economic development of companies, especially SMEs, of the member states, by providing better insights to promote the development and characterization in the clinical settings of biomaterials as well as more comprehensive view of their properties.

Raising awareness on sharing and big data awareness and interest among the biomaterial research community and industry
BIOMATDB will be fully open access and reliant on open-source computational tools, which will raise awareness on the meta-analysis of a large number of past clinical trails to reveal numerous novel findings. For example, at least one symposium will be organized around the topics of ‘big-data and biomaterials’ within a large international tissue engineering or biomaterials congress, hoping to create an international group of biomaterials scientists with deep interest in the topic.Support for the suppliers of biomaterials (companies, especially SMEs) in choosing and facilitating market access
Biomaterials often have applications beyond their originally planned application and beyond their originally targeted industry. Through the BIOMATDB database generally and specifically through a large pool of metadata, further application areas can be identified more easily. It’s of utmost importance to better equip the ecosystem with better tools and data to foster innovation and close the cap to other parts of the world.

Increase the public acceptance of Artificial Intelligence and related modern technologies and its benefits
An additional indirect impact of the project is to increase and improve the public perception of the advantages of combining and exploiting heterogenous data repositories with AI and machine learning algorithms for a beneficial purpose to support in crisis- and healthcare-related challenges for society. Attention will be paid towards outlining the technical capabilities of the platform and their impact on improving the discoverability, availability and quality of the offered products and services.
Impact on the competitiveness of the European Union in the field of biomaterials, medical devices and biomedical engineering
Advanced information technology applications show the potential to dramatically increase patient safety by cross-referencing multiple data sources. By creating the BIOMATDB database, clinicians can directly access relevant information about e.g.: adverse drug interactions at the point of care and make better informed treatment decisions.
Project Structure
WP1 MANAGE: Project Management, Consortium Coordination, Data & IPR, and Reporting
The main objective of WP1 is the coordination and management of the consortium partners as well as tackling data-related issues, legal aspects and IPR management.
WP2 DEFINE: Knowledge, Requirements, Process, Practices, Meta Use Cases, and Data Sources
The main objective of WP2 is the compilation of existing knowledge and insights from former projects and external sources for material databases, marketplaces and digital advisors.
WP3 CONCEPT: Features, Technical System, Advanced database, Analysis Tools, Marketplaces, Digital Advisors
The main objective of WP3 is the conceptualization of a target-group-oriented, advanced biomaterial database and intelligent data processing tools (search, decision making, analyses, visualisations)
WP4 DEVELOP: Database System, Data Analysis Tools, Web Applications, Backend Modules, and Multilingual Frontends
The main objective of WP4 is the development of the core technical database system (backend and frontend) to derive the data tools and marketplace applications for the different demonstration cases.
WP5 DEMONSTRATE: Deployment, Validation, User Tests, Training Activities, Feedback Gathering, and Optimisation Requests
The main objective of WP5 is the demonstration, testing and validation of the developed Biomaterial Database and all related data analysis and visualisation tools for the different demonstration cases.
WP6 DISSEMINATE: Communication, Dissemination, Business Models, Strategy Creation, and Exploitation
The main objective of WP6 is the communication and dissemination of project-related information and the mobilisation of identified stakeholders to achieve a successful uptake and usage of the BIOMATDB solution.
Deliverables
D2.1 Knowledge compilation and structural material collection
D2.2 Stakeholder Survey
D2.4 Updated stakeholder survey
D3.2 Database, data processing methods, tools and web application marketplace and specifications (Phase 1)
D4.2 Database and marketplace application frontend and backend (Phase 1)
D4.3 Database and marketplace application frontend and backend (Phase 2)
D4.5 Online knowledge base (KB) with manuals, FAQ, and screencast video tutorials
D5.1 Demonstration plan and test system
D6.1 Project website with social media channels and communication plugins
D6.2 DEC plan and DEC materials (Package 1)
D6.3 DEC plan update and DEC materials (Package 2)
Deliverables
D2.4 Updated stakeholder survey
D4.3 Database and marketplace application frontend and backend (Phase 2)
D4.5 Online knowledge base (KB) with manuals, FAQ, and screencast video tutorials
D6.3 DEC plan update and DEC materials (Package 2)