We want to hear from you!
Medical implants for human applications are subject to high legal requirements to ensure safety and performance. As of May 26, 2021, the new European Medical Device Regulation (EU)2017/7458 MDR) [1], [2] also applies to implants. This article outlines the regulatory requirements for medical implants in Europe as well as the connected procedure.
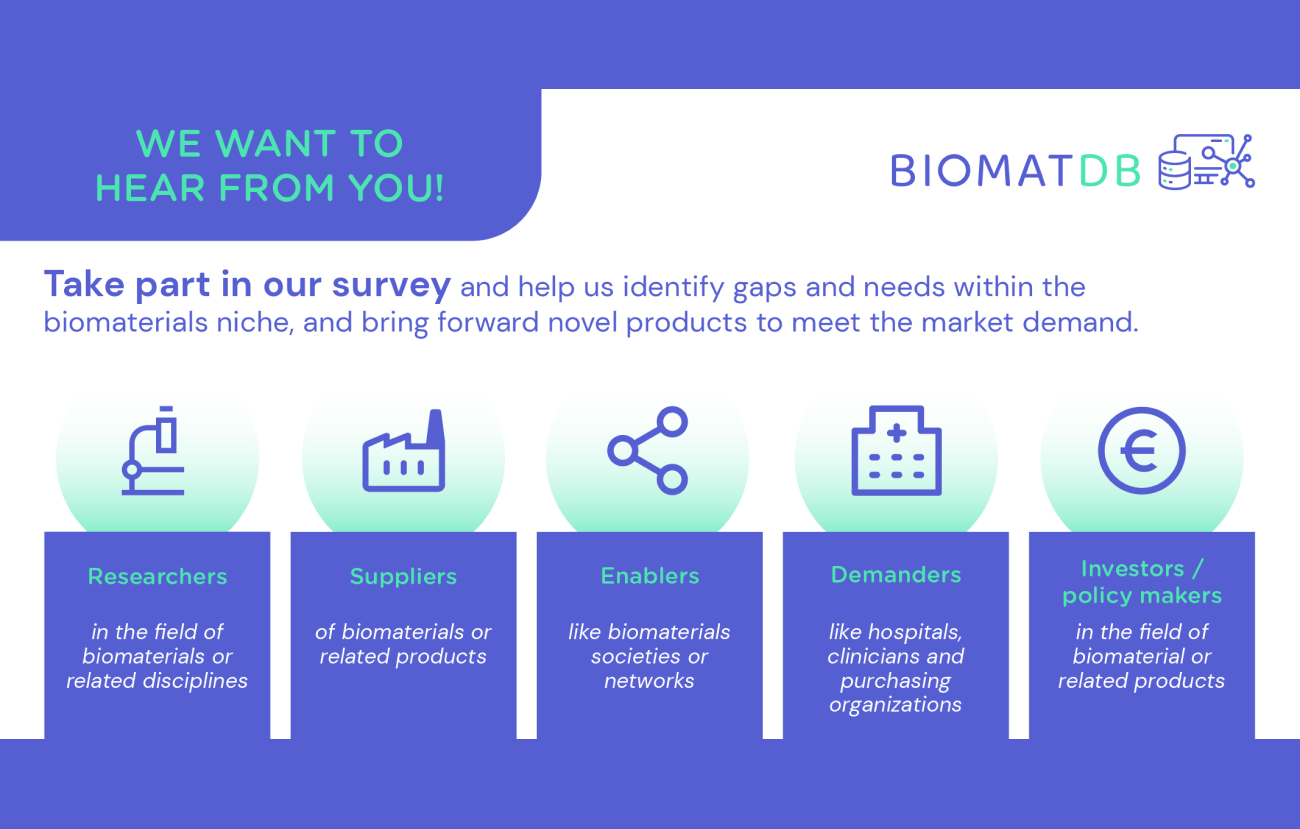
The BIOMATDB project develops a biomaterials database and marketplace. In doing so, we place particular importance on tailoring these solutions to the needs and requirements of their potential users. Therefore, we are conducting surveys with stakeholder groups that will benefit from the advantages of a database and marketplace in the future. The results from these surveys will be evaluated and taken into account for the development of the biomaterials database and marketplace. More information about the BIOMATDB solutions can be found in the description of our project objectives.
If you belong to one of the following groups, we would invite you to share your inputs with us:
- Researchers in the field of biomaterials or related disciplines
- Suppliers of biomaterials or related products
- Enablers like biomaterials soctieties or networks
- Demanders like hospitals, clinicians and purchasing organisations
- Investors & Policy makers in the field of biomaterials or related products
You can find the surveys here:
Researchers: https://ec.europa.eu/eusurvey/runner/BIOMATDBSurvey22-Researchers
Suppliers: https://ec.europa.eu/eusurvey/runner/BIOMATDBSurvey22-Suppliers
Enablers: https://ec.europa.eu/eusurvey/runner/BIOMATDBSurvey22-Enablers
Demanders: https://ec.europa.eu/eusurvey/runner/BIOMATDBSuvey22-Demanders
Investors & Policy makers: https://ec.europa.eu/eusurvey/runner/BIOMATDBSurvey22-Investors-Policy
We are looking forward to hearing your opinion!
References (English)
[1] European Parliament and the Council of the European Union, Regulation (EU) 2020/561 amending Regulation (EU) 2017/745 on medical devices as regards the date of application of certain of its provisions. 2020. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0561&from=EN, accessed on 05.10.2022.
[2] European Parliament and Council of the European Union, Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 concerning medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC. 2017, pp. 1-228. Available at: https://eur-lex.europa.eu/eli/reg/2017/745, accessed on 05.10.2022.
[3] Prinz, T. (2022), “Approval of medical implants under the European Medical Device Regulation (MDR),” RSPONSE Partnership for Innovation in Implant Technology. Available at: https://www.vde.com/resource/blob/2182192/ab8a8c083e21c40e4eecea6d3a86b217/positionspapier-data.pdf, accessed on 05.10.2022.
[4] BVMed – Bundesverband Medizintechnologie e.V., ed., Classification list for medical devices. Berlin, 2014.
[5] Medical Device Coordination Group, MDCG 2021-24 Guidance on classification of medical devices. 2021. Available at: https://health.ec.europa.eu/system/files/2021-10/mdcg_2021-24_en_0.pdf, accessed on 05.10.2022.
[6] Medical Device Coordination Group, MDCG 2019-11 Guidance on Qualification and Classificationof Software in Regulation (EU) 2017/745 – MDR and Regulation (EU) 2017/746 – IVDR. 2019. Available at: https://ec.europa.eu/docsroom/documents/37581, accessed on 05 Oct. 2022.
[7] Medical Device Coordination Group, MDCG 2020-5 Clinical Evaluation – Equivalence. A guide for manufacturers and notified bodies. 2020. Available at: https://health.ec.europa.eu/system/files/2020-09/md_mdcg_2020_5_guidance_clinical_evaluation_equivalence_en_0.pdf, accessed on 05.10.2022.
[8] Medical Device Coordination Group, MDCG 2021-6 Regulation (EU) 2017/745 – Questions & Answers regarding clinical investigation. 2021. Available at: https://health.ec.europa.eu/system/files/2021-04/mdcg_2021-6_en_0.pdf, accessed on 05.10.2022.
[9] Medical Device Coordination Group, MDCG 2019-8 v2 – Guidance document – Implant Card relating to the application of Article 18 Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices. 2020. Available at: https://ec.europa.eu/health/system/files/2020-09/md_mdcg_2019_8_implant_guidance_card_en_0.pdf, accessed on 05.10.2022.
[10] Medical Device Coordination Group, MDCG 2021-11 Guidance on Implant Card – ‘Device types’.2021. Available at: https://ec.europa.eu/health/sites/default/files/md_sector/docs/md_2021-11_en.pdf, accessed on 05.10.2022.
[11] Medical Device Coordination Group, MDCG 2019-7 Guidance on Article 15 of the Medical Device Regulation (MDR) and in vitro Diagnostic Device Regulation (IVDR) regarding a ‘person responsible for regulatory compliance’ (PRRC). 2019. Available at: https://ec.europa.eu/docsroom/documents/36166, accessed 05 Oct. 2022, accessed on 05.10.2022.
[12] European Commission, ed, “The ‘Blue Guide’ on the implementation of EU products rules 2016.” July 26, 2016. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/ PDF/?uri=CELEX:52016XC0726(02)&from=BG, accessed on 05 Oct. 2022.
[13] Global Harmonization Task Force, Role of standards in the assessment of medical devices.
- Available at: http://www.imdrf.org/docs/ghtf/final/sg1/procedural-docs/ghtf sg1-n044-2008-standards-in-assessment-of-medical-devices-080305.pdf, accessed on 05.10.2022.
[14] S. Hallscheidt, N. Adomeit, T. Manske, and J. U. Hopf, “1×1 der Normung – Ein praxis-orientierterLeitfaden für KMU.” May 2019. accessed Sep 19, 2019. Available at: https://www.din.de/blob/64110/b5d8d57f3b7e6866233201bf45aad388/1×1-data.pdf, accessed on 05 Oct. 2022.