Biomaterials data: where to find it and difficulties in its interpretation
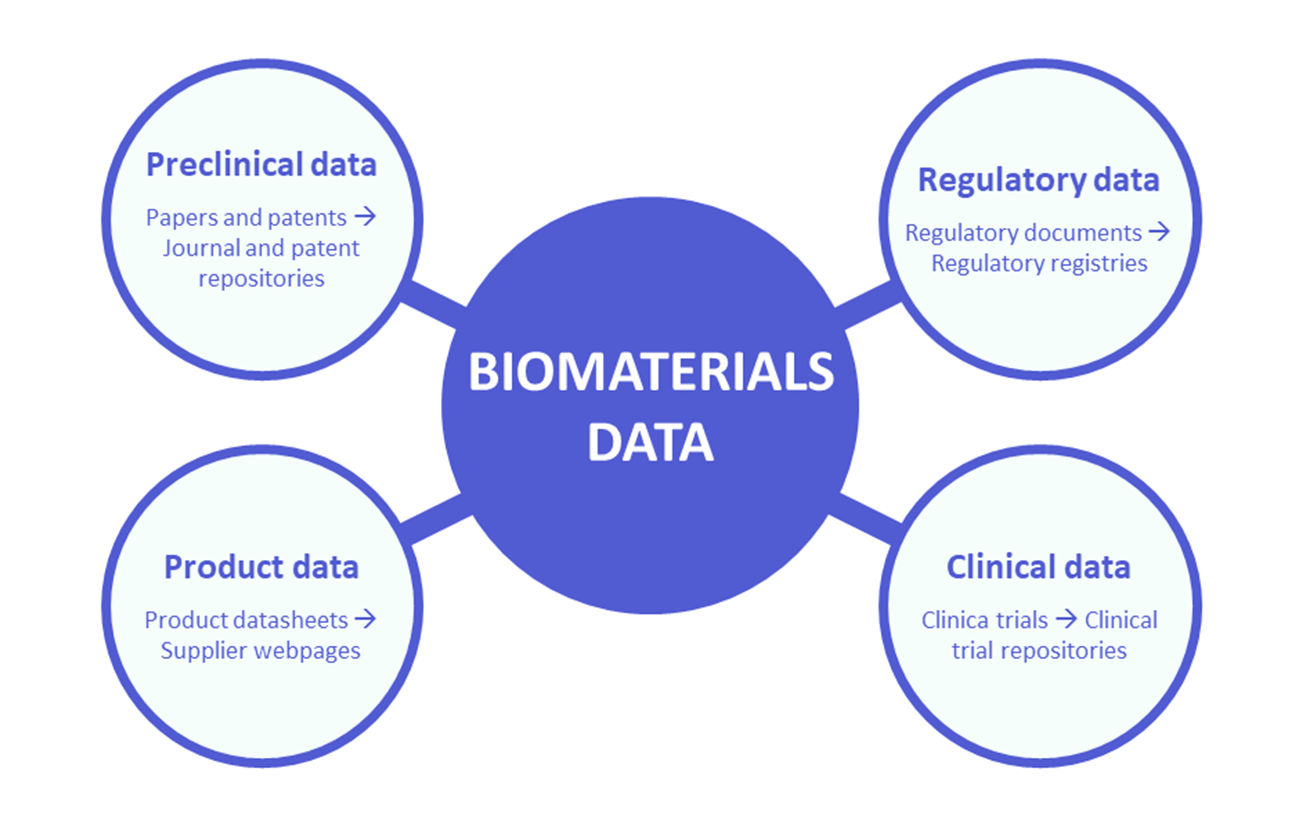
This article aims to provide a concise and clear overview of data derived from the biomaterial lifecycle, sources in which the data can be found, and their main features, capabilities and limitations (Figure 1). This overview provides an important basis for the development of a database and marketplace for biomaterials.
Materials which interact with living systems to improve people’s quality of life are known as biomaterials. The development of these kinds of materials produces a huge amount of data distributed among different data sources, making it difficult to stay up to date and gain a comprehensive understanding. Several open access tools try to facilitate this process; however, they are few in number and limited to very specific areas. In the following article, the types of data derived from the development of biomaterials, the main sources and their main capabilities and limitations will be identified.
Biomaterials are known as materials that have been engineered to direct the course of therapeutic or diagnostic procedures by interactions with components of a living system [1]. The biomaterials field has lately gained a lot of interest because of the materials’ wide range of applications in medicine [2]. They are key components of medical devices and advanced therapies that benefit patients and society by improving long-term healthcare outcomes and patients’ quality of life [3].
In this constantly growing field, research is exponentially increasing. In PubMed, a free resource supporting the search and retrieval of biomedical and life sciences literature, the number of results per year for the search of the term “biomaterial” has increased ten times in the past 20 years. Some of the materials investigated in these publications are patented, and subjected to strict regulations so that they can eventually become part of an approved medical device.
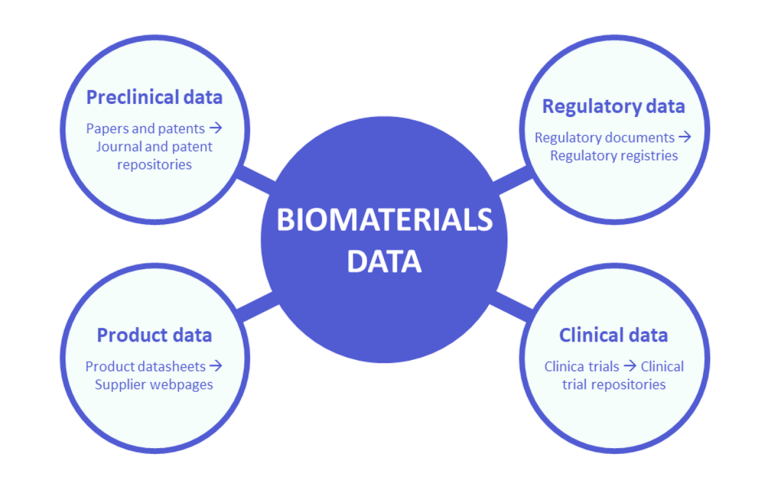
The development and lifecycle of biomaterials produces a vast amount of heterogeneous data. This includes, among others, data derived from prototype development, preclinical evaluation, clinical trials, or post-market surveillance, as well as data provided by manufacturers in the form of product data sheets. All this data is fragmented and distributed among different documents and sources (Figure 2). Understanding the types of data related to biomaterial development, the types of documents where this data is found and the sources to access these documents is a fundamental step when pursuing the goal of consolidating, centralising and organising this data.
Preclinical data
A vast amount of biomaterial information can be found in investigational preclinical data that can be obtained from research articles. The most common way of accessing a bulk quantity of biomaterials articles is through scientific indexing and archiving services. Secondly, preclinical data can also be obtained from patents, which are registered in specialized patent databases. A quick summary of both types of tools can be found in Table 1 and Table 2, respectively. While these access points represent an excellent source for a majority of the data gathered in the domain, extracting information from such text documents in an automated way remains a major challenge. Another difficulty is that relevant articles and patents can be found across a wide variety of disciplines, making it difficult to get a comprehensive overview of the information.
Table 1: Main journal repositories, their specifications and results recorded when searching for the terms biomaterial, medical material or tissue engineering
|
|
PubMed |
Scopus |
Web of Science |
|
Records |
773K |
470K |
107K |
|
Metadata download |
10K records |
20K records |
10K records |
|
Access |
Open access |
Requires registration |
Requires registration |
|
Sponsor |
NIH |
Elsevier |
Clarivate |
|
URL |
Table 2: Main general patent databases, their specifications and results recorded when searching for the terms biomaterial, medical material or tissue engineering
|
|
Google Patents |
Espacenet |
Patent Scope |
LENS |
USPTO |
Scopus Patents |
|
Records |
128K |
1,9M |
114K |
1,78M |
30K |
2,1M |
|
Metadata download |
25K records |
500 records |
10K records |
1K records |
No |
No |
|
Access |
Yes |
Yes |
Yes |
Yes |
Yes |
Requires registration |
|
Sponsor |
|
EPO |
WIPO |
Cambia |
USPTO |
Elsevier |
|
URL |
Clinical data
A fundamental step in the development of a biomaterial is to demonstrate both its efficiency regarding its specific purpose and its long-term safety in the corresponding clinical scenario. For that, clinical trials are a highly valuable source of information in medicine and especially in the biomaterials field. Clinical trial repositories (Table 3) allow to access detailed information about clinical reports, specifically data related to the clinical setup (i.e., number of participants, criteria for the selection of patients, surgical procedure, etc.) and detailed data about the results, when available. Having said this, these data sources present limited information about the medical product employed in the clinical trial (e.g., mechanical and physicochemical properties, composition, sterilization procedure, etc.), which makes it difficult to relate the results to the features of the biomaterial.
Table 3: Main clinical data repositories with total size, where available
|
Name |
URL |
Description |
Size |
|
EU Clinical Trials Register |
The European Union (EU) Clinical Trials Register enables protocol and result exploration of interventional clinical trials conducted in the EU and the European Economic Area (EEA) and clinical trials conducted outside the EU / EEA linked to European paediatric-medicine development. |
43K |
|
|
ECRIN |
The Clinical Research Metadata Repository, an online tool to help researchers explore data linked to clinical research study, and to obtain information on the accessibility of those results. |
>10K |
|
|
ClinicalTrials.gov is a database of global, privately and publicly funded clinical studies. |
424K |
||
|
TrialSearch |
The Clinical Trials Search Portal provides access to a central database and links to the full original records. |
- |
|
|
OpenTrials |
Collaboration between Open Knowledge International and Dr Goldacre (University of Oxford DataLab), aiming to locate, match and share all publicly accessible data/documents, on all trials conducted, on all medicines/treatments, globally. |
- |
Regulatory data
The final goal of an engineered biomaterial is to become part of a medical product that will pass the approval procedures of regulatory agencies. To achieve the approval of medical products, regulatory documentation containing evidence of their intended clinical use is required and needs to be presented to regulatory agencies (i.e., Food and Drug Administration for the U.S. and European Medicines Agency for Europe). The content of this kind of approval documents depends on the type of product and the extent of its differences to similar approved products. In addition, one fundamental aspect about approved biomaterials is that their long-term performance and adverse effects must be monitored and reported. For that, regulatory agencies ask manufacturers to submit post-market surveillance reports for their products.
In the case of medical devices approved by the FDA, the different kinds of documentation are registered among several databases that can be found at Medical Device Databases | FDA. Similarly, medical devices on the European market are being registered in the EUDAMED database (Devices/SPPs – EUDAMED). Although these tools help to manage the regulatory documentation of medical products, similarly to what can be observed with clinical trial repositories, the specific biomaterial composition is not well determined, which makes it difficult to correlate the biocompatibility results of medical devices with the biomaterials they are composed of.
Product data
Relevant data about biomaterial-related products can be found in commercial product datasheets, both for the final medical product and for suppliers of raw materials. However, in the case of raw material suppliers, the information about this kind of commercial product is not well centralised and compiled in specific tools covering the biomaterials field. Biomaterial suppliers are mostly found among material marketplaces that cover a large amount of industrial material applications, with the lack of specialization making it difficult to find relevant information. One exception is knowde – The marketplace for ingredients, polymers and chemistry, which offers a separate section for materials employed for medical applications. However, the main limitation of knowde is that the marketplace mostly focuses on polymers, and it does not collect key aspects of the materials such as detailed information about the sterilisation process or about which characteristics qualify them as medical grade biomaterials. In addition, following the same trend as clinical and regulatory data, the connection of medical products and their biomaterials composition is not well defined due to confidentiality issues.
Biomaterial databases
To facilitate the understanding of biomaterial data and, subsequently,their development, the construction of specialized databases in this field is required. As these specialized databases reduce the amount of raw data, they can be used to provide advanced search systems or even visualization tools that help to interpret the information. In the biomaterials field, specialized databases are still limited, and only a few open access tools can be found (Table 4).
Table 4: Specialized databases and results recorded by searching for the terms biomaterial, medical material or tissue engineering
|
Name |
URL |
Description |
Size |
|
Database of Experimental Biomaterials and their Biological Effect (DEBBIE) |
EU Horizon 2020 funded project which developed an open access database automatically curated from PubMed abstracts |
344K |
|
|
Compendium for Biomaterial Transcriptomics (cBiT) |
The first repository that offers biomaterial-based transcriptomics data together with relevant biomaterial metadata. |
28 |
|
|
3D Printing Database |
Database of articles involving 3D printing in the field of tissue engineering and regenerative medicine, systematically collating parameters that dictate material 3D printing. |
807 |
The biomaterial databases presented in Table 4 enable a more comprehensive understanding of some aspects related to the biomaterials field, nevertheless, they also have their limitations.. In the case of the DEBBIE database, the analysis is restricted to abstracts and may thus limit the data capture that the database can achieve. cBiT and the 3D Printing Database, only cover specific areas of the biomaterials field and a small volume of articles. Relevantly, all these databases are operating on restricted investigational data and therefore, the correlation between clinical, regulatory and product data is still missing. The BIOMATDB project aims to create an open access database analysing text data of whole documents from all the previously mentioned data sources, with the aim of encompassing and linking information from each phase of the lifecycle of a biomaterial, from the first steps of their development to the commercialization of a medical product.
Biomaterial marketplaces
When searching for marketplaces specialised on raw biomaterials products and suppliers, only two results were obtained next to Knowde: the Biomaterials Store, which provides grafting materials, sutures and instruments of different companies in the UK for dental professionals (https://www.thebiomaterialstore.co.uk), and the Bonezone, which is a customer-centric media company exclusively serving the global orthopaedic market (Supplier Directory – BONEZONE (bonezonepub.com). These two marketplaces are niche-specific, collecting only suppliers and products from two specific sectors in the biomaterials field and focusing more on sales than on collating biomaterial features. In the BIOMATDB project, the consortium aims to provide a web-optimised information marketplace of raw biomaterials and their suppliers and tries to describe which biomaterials are employed in specific medical products to support companies in their decision-making processes.
Conclusion
The lifecycle of a biomaterial-based product produces large amounts of information in each stage of its development that is fragmented among different data sources and not well connected, mainly due to confidentiality issues and missing information. Some existing databases tried to facilitate this process, but they are still limited to investigational data. To have a more comprehensive understanding of biomaterial development, a fundamental first step is to recognize the type of information and documents derived during the biomaterial-based product development and the sources to find them. Having a detailed overview of these data sources could enable the production of new biomaterials databases with tools that allow linking the different types of documents in the biomaterials field. This is exactly what the BIOMATDB project is attempting to do: The BIOMATDB database aims to provide different tools to compare and link this information, specifically focusing on the physicochemical and biocompatibility data, while the BIOMATDB marketplace should centralise biomaterial suppliers data and thereby facilitate the production of biomaterial-related products.
References
[1] David Williams, X. Z (2019). Definitions of Biomaterials for the Twenty-First Century – 1st Edition. Elsevier.
[2] Fergal Donnelly (2015). European perspectives on biomaterials for health. Eur. Wound Manag. Assoc. J. 15, 54–58.
[3] Chandra Sharma (2019). Biointegration of Medical Implant Materials – 2nd Edition. Elsevier.